
Urinary tract infections (UTIs) are among the most common type of healthcare-associated infections, resulting in nearly 13,000 deaths each year. Nearly 75% are associated with a urinary catheter.
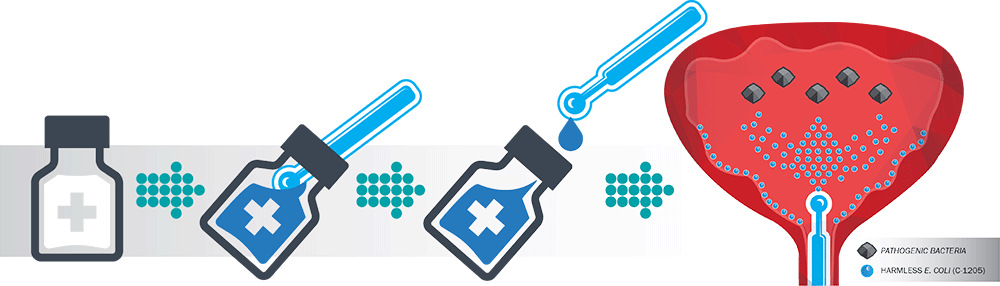
Atterx has developed C-1205, an innovative product that uses bacterial interference to address the growing problem of CAUTIs. C-1205 replaces the traditional lubricant used to coat urinary catheters prior to their insertion into the patient. C-1205 is a lyophilized (freeze-dried) powder containing a harmless bacterium and a gelling ingredient, that can be stored for many years with no loss of activity. The product is reconstituted with water when needed, forming a thick gel for catheter insertion. Insertion of the catheter into the patient introduces the bacteria into the bladder where it forms a beneficial colony that prevents UTIs.
Cost of hospital-associated UTIs was nearly $1 billion in 2013. Costs are expected to climb to nearly $3 Billion by the end of the decade.
The efficacy of C-1205 in reducing the frequency of CAUTIs has been studied with two different strains of E. coli: E. coli 83972 and E. coli HU2117. Both strains are harmless. C-1205 uses Atterx’s formulation of E. coli HU2117.
Non-Colonized Patients
Colonized Patients
E. coli 83972
E. coli HU2117
Both strains safely colonize the bladder of patients. Colonized bladder results in significant reduction in CAUTIs.

The FDA has approved a Phase I trial using Atterx C-1205. This will allow rapid entry into a Phase II trial. The Phase I/II trial will involve 40 patients in four groups to confirm the safety of the product.
The trials will determine rates and time to colonize bladder, evaluate maintenance in the bladder after removal, investigate the presence of bacteria in urine, and evaluate the efficacy of C-1205 to prevent infection.